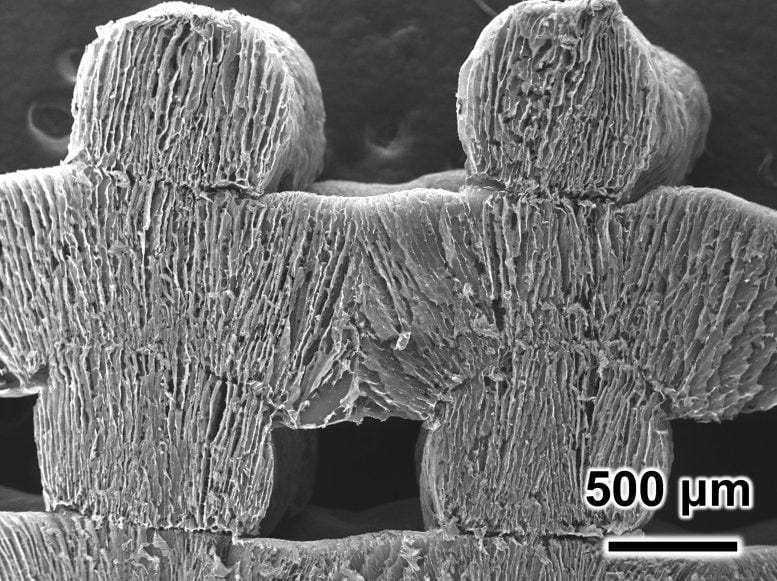
Hello! This is Everything Is Amazing, a newsletter about science, curiosity & wonder, and the counterintuitive benefits of doing things for no damn reason. Today’s a quick one, at least by my rambling standards. While this newsletter is mostly concerned with diving into big topics like mind-bending illusions, fake maps, semi-impossible colours and the restless innards of our planet, I’m also going to be making more of an effort to point you towards exciting scientific breakthroughs that inspire a bit (or a lot) of hope for our complicated and messy world, as I said here. Yesterday, thanks to legendary blogger Jason Kottke, I spotted an absolute corker. This is an extreme closeup of a type of aerogel - a synthetic, porous, sponge-like material, in this case made from carbon nanotubes and cellulose nanofibers, constructed with a 3D printer. In a test reported in the journal ACS Energy Letters and picked up by SciTechDaily, scientists used it to do something absolutely astonishing:
In other words, this is a material made of one of the most common substances on earth, purifying seawater using nothing but the power of the sun. Allow me to excitedly unpack the implications of this for a second. Even though the surface of our planet is 71% water and there are truly vast amounts of the stuff in our oceans, the fraction that’s drinkable (potable) is absolutely tiny. After discounting the freshwater locked up in glaciers and ice, it amounts to around 1.2% of the Earth’s entire supply of water. (It’s a little head-spinning how relatively little non-frozen freshwater there is: southern Siberia’s Lake Baikal is estimated to contain around 19% of it - nearly a fifth of the world’s supply!) Water scarcity is an everyday problem in many parts of the world - and as our planet continues to heat up, it’ll get worse, especially when it comes to supplying the needs of agriculture. Worldwide, we already don’t have enough water, and yet we’re still going to need a lot more. Enter desalination - the stripping out salts and impurities from seawater to make it potable. It’s already a viable technology on a big scale, and it’s becoming increasingly cheap, as Hannah Ritchie (Head of Research at Our World in Data) explains here: But even a few dollars a year can put it outside the budgets of the poorest - and these techniques still require energy (often a great deal of it) in the form of electricity. That makes it inaccessible to many, and also to agriculture running on the thinnest of margins. Perhaps not so with this aerogel, if I’m understanding all this correctly. If (admittedly a really big if) this material could be affordably made at scale, and if it’s durable enough - which you’d certainly expect a carbon-nanotube-containing structure to be - and it doesn’t degrade into a pollutant over time in the way that microplastics have arisen, then this is potentially a vast step forward towards fixing the world’s future freshwater shortages. (Another question I have: if this process creates drinkable water from seawater, what about from polluted freshwater? What about all the contaminated water supplies that wreak havoc in water-stressed countries? Could it work for them too?) By making desalination so accessible without the need for power beyond a steady supply of sunlight, it could take our planet’s limited supply of fresh water (which I previously wrote about here) and - what? Even going some way towards doubling it would be utterly transformational, and ease a great deal of human suffering. Thanks to this arresting image by the United States Geological Survey, you can see how much more water is available: (That bigger drop, comprising all the world’s water, contains an unimaginable 332 million cubic miles of water.) This aerogel breakthrough is a truly remarkable technical achievement. And a revolutionary one? I very much hope so. It certainly feels like a “whew, we really are living in the future” moment. One thing to bear in mind, though: desalination creates a lot of salt & brine polluted with chlorine and copper (plus trace elements). Presumably, the aerogel method would create something similar, so the safe disposal, or ideally upcycling, of this by-product would be needed to render the whole process sustainable. One tantalizing possibility: batteries! The sodium extracted from sea salt can be used to make sodium-ion batteries, already set to command nearly a quarter of the stationary storage market in the next half-decade. A technology that helps solve one problem while also creating a by-product that helps solve another? Be still my beating nerdy heart. So - very early days. But all rather exciting. I’ll keep you posted. - Mike |